News & Resources
Neuroimaging Biomarkers for Friedreich Ataxia: A Cross-Sectional Analysis of the TRACK-FA Study
Neuroimaging Biomarkers for Friedreich Ataxia: A Cross-Sectional Analysis of the TRACK-FA Study Objective Our study aims to quantify differences in the brain and spinal cord between individuals with Friedreich's ataxia and healthy controls. This includes stratification by age and disease stage, and for the first time, the inclusion of young children.
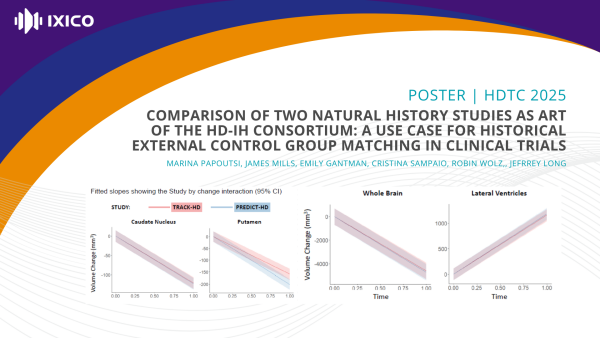
Comparison of two natural history studies as part of the HD-IH consortium: a use case for historical external control group matching in clinical trials
External control groups can support regulatory decision-making in clinical trials, especially rare diseases. In Huntington’s disease (HD) there is a wealth of historical data, but matching historical data to clinical trials is challenging.
International Women's Day 2025
International Women's Day (IWD) has been around for over a hundred years, as have many of the issues still impacting women's advancement. Since 1911, IWD belongs to all who care about women's equality, celebrating women's achievement, raising awareness about discrimination and taking action to forge gender parity.

Contract Wins in Alzheimer’s and Huntington’s Disease
27 February 2025 - London, UK. IXICO plc (AIM: IXI, “IXICO” or “the Company”) a global leader in neuroscience imaging, using its AI-driven platform to help advance therapy research in neurological disorders, today announces that it has signed a new commercial contract for a global Alzheimer’s Disease (AD) imaging analysis with a leading biotechnology company, and secured an additional project in Huntington’s Disease (HD) with an existing partner.

Huntington’s Disease consortium further validates IXICO’s AI imaging platform
12 February 2025 - London, UK. IXICO plc (AIM: IXI, “IXICO” or “the Company”) a global leader in neuroscience imaging, using its AI-driven platform to help advance drug development in neurological disorders, today announces, the generation of further evidence verifying the superiority of the ‘IXIQ.Ai’ analysis platform in the development and validation of imaging biomarkers for Huntington’s Disease (HD), in conjunction with the Huntington’s Disease Imaging Harmonisation (“HD-IH”) consortium.

Commercial agreement with PETNET Solutions Inc, a Siemens Healthineers Company
16 December 2024 - London, UK. IXICO plc (AIM: IXI, “IXICO” or “the Company”) a global leader in neuroscience imaging, using its AI-driven platform to help advance therapy research in neurological disorders, today announced a commercial agreement with PETNET Solutions Inc, a Siemens Healthineers company (“PETNET”) to supply diagnostic imaging agents to IXICO. This new offering is an integral part of IXICO’s expansion strategy to serve its growing customer base with the latest technologies in neuroimaging.

US Client Clinical Trial Contract Win
10 December 2024 - London, UK. IXICO plc (AIM: IXI, “IXICO” or “the Company”) a global leader in neuroscience imaging, using its AI-driven platform to help advance therapy research in neurological disorders, today announces that it has signed a contract with a new US headquartered client to provide imaging services for a Phase 2 Huntington’s Disease clinical trial.
33-40 of 183 results