Radiological reads
Radiological reads
IXICO provides central neuro radiological reading of MRI scans to support the assessment of subject eligibility and the ongoing monitoring of drug safety.
We work with a world renowned network of neuroradiologists from internationally recognised neurology centres to provide the specialist knowledge required to accurately and consistently read MRI scans.
As such, IXICO is able to support very large studies, screening 1,000s of subjects and reading 10,000s of MRI scans to assess drug safety.
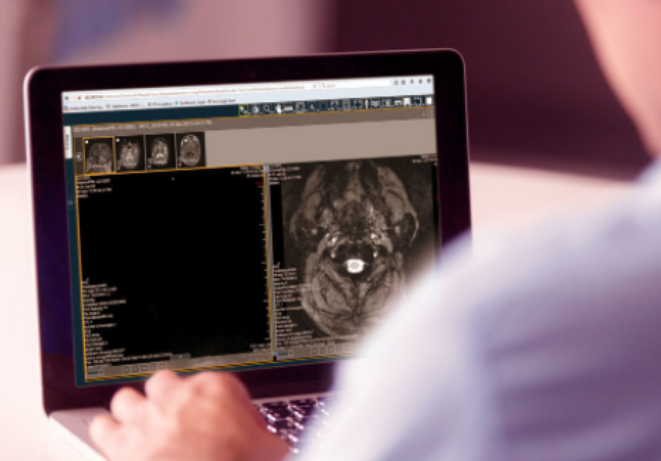
Reading platform
Our TrialTracker™ platform provides a standardised, compliant and efficient workflow for capturing visual read results of MRI, SPECT, PET and CT scans, supporting single, over-read and double adjudicated read paradigms.
MRI images are read using an integrated radiological grade DICOM viewer and the read results are reported within an electronic read form, pre-configured to meet the reporting requirements for the study.
TrialTracker ensures that we meet turnaround times required for time critical central assessments such as for evaluating subject eligibility and safety, reporting results of central assessments to investigators, sponsors and drug safety monitory boards.
Our experienced radiology and nuclear medicine readers are continuously trained in the process for reading scans. For example, our readers complete a number of test cases selected to replicate typical variability in the target patient population. The results of this training set allows us to ensure reader variability is within acceptable tolerances.
Platform access for safety monitoring
We can provide our biopharma clients’ drug safety monitoring boards with controlled and defined access to image data and central read results for review of any eligibility and safety findings.
Neuroradiology Consultancy
We support our biopharma clients to define:
- the radiology criteria for subject eligibility and any radiological scoring cut offs
- the thresholds for significant safety findings that are monitored during the study
- the action that should be taken should such a finding develop
- the schedule of additional MRI scans to monitor patients with positive safety findings.

Nuclear medicine visual reads
IXICO provides centralised visual SPECT and PET reads for clinical trials including but not limited to:
- 18F fludeoxyglucose (FDG)
- 18F-florbetapir (Amyvid™, Eli Lilly)
- 18F-flutemetamol (Vizamyl™, GE Healthcare)
- 18F-florbetaben (NeuraCeq™, Piramal)
- 123I-Ioflupane (DaTscan™, GE Healthcare).
With our network of eminent neuroradiologists, IXICO is able to provide the expertise and training only available in specialist centres required to accurately and consistently read nuclear medicine scans.
We also work closely with tracer manufacturers on a number of clinical trials and also as part of the IMI AMYPAD project.