The selection of participants at risk of cognitive decline in clinical trials, known as trial enrichment, increases the probability of trial success. It is estimated that by 2050, 153 million people worldwide will be living with a type of dementia. Hence, innovative trial recruitment strategies are necessary to accelerate treatment development.
Here we present a deep-learning framework for trial enrichment for Alzheimer's Disease that uses a combination of neuroimaging and clinical/demographic variables as inputs. The framework is designed with built-in redundancy allowing the system to work effectively with missing inputs.
Methods
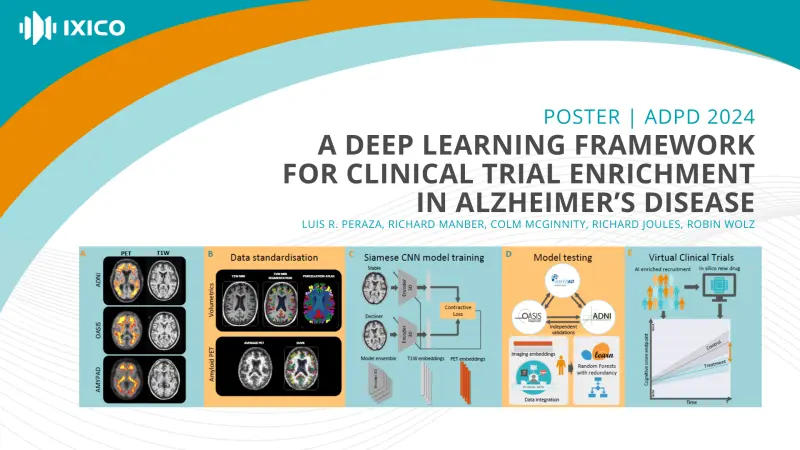
We employed T1-MR and amyloid-PET images from ADNI, OASIS-3 and AMYPAD repositories. Images were pre-processed by validated pipelines for volumetric and amyloid PET SUVR estimation [1-4].
Our framework comprises two phases: Firstly, Siamese convolutional neural network (CNN) encoders were trained with decline/stable targets and neuroimages as inputs. Secondly, a battery of random forest classifiers were trained with CNN outputs, neuroimaging derivatives, and demographics/clinical data inputs and decline/stable targets.
Results
- We independently validated our framework using ADNI, OASIS and AMYPAD datasets.
- Across the analysed datasets, the percentage of participants who cognitively declined was of 15%, 30% in MCI participants, highlighting the importance of this work.
- The framework reached an 83% mean balanced accuracy across all independent datasets.
- Our framework proved it can potentially save 72% of PET imaging costs at recruitment time by using MRI based prediction followed by PET imaging confirmation.
- The VCT experiments showed that our framework selects participants who experience accelerated decline and who are at early disease stage.
Presented at ADPD 2024


