External control groups can support regulatory decision-making in clinical trials, especially rare diseases. In Huntington’s disease (HD) there is a wealth of historical data, but matching historical data to clinical trials is challenging.
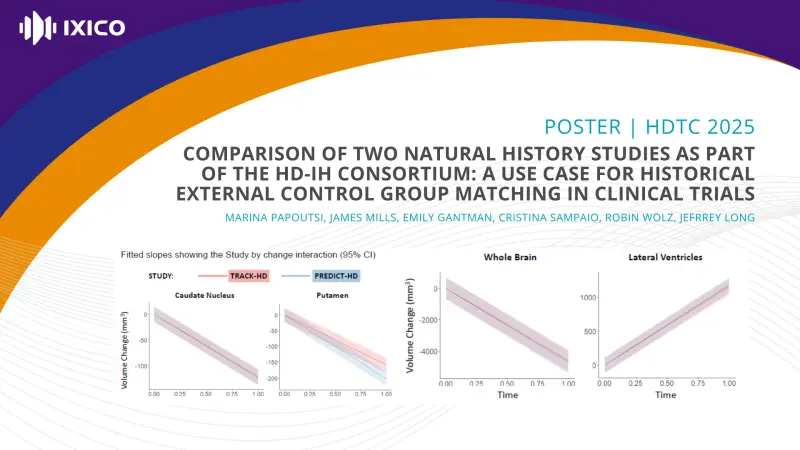
Here, we show how historical data from two natural history studies, TRACK-HD and PREDICT-HD, have sufficiently similar sub-populations to allow their merging and use as an external comparator group. To evidence this we selected matched samples from the two studies using propensity scores and compared groups using change over time in caudate, putamen, whole brain and lateral ventricles volume, and composite Unified HD Rating Scale (cUHDRS), Symbol Digit Modalities Test (SDMT), Total Motor Score (TMS) and Total Functional Capacity (TFC).
Method
Using data from the HD Imaging Harmonization (HD-IH) consortium we selected 87 participants from TRACK-HD using criteria: 25-65 years old, CAG repeat length (CAG) 40-50 and HD-ISS Stage 2 or 3 and TFC > 10 (Tabrizi et al, 2022). These criteria are similar to a putative clinical trial.
Participants also had to have a 2-year follow-up MRI scan, because in PREDICT-HD most scans occurred bi-annually. Using very different time-intervals is not recommended when matching studies, even if annualized. This is because of differences in sensitivity across time either due to algorithm bias in the case of volumetry or practice effects in the case of clinical scores.
Summary
For HD-ISS Stage 2 and early Stage 3 participants there was no significant effect of study on change for any of the eight outcomes measured here (all p > 0.05 uncorrected). The two matched groups had similar trajectories over 2 years.
Our work therefore shows that data from the two studies are sufficiently similar to be merged and considered as an external comparator group in clinical trials.


