IXI™ is IXICO’s proprietary, AI-enabled neuroimaging technology platform for precision medicine, using machine learning and deep learning to drive clinical trial imaging and biomarker science insights in neurological disease research.
The platform integrates standardised high-quality image data capture, image reading, advanced analysis, and regulatory-compliant reporting of clinical data, enabling the biopharma industry to develop new medicines and diagnostic measures via a single end-to-end platform.
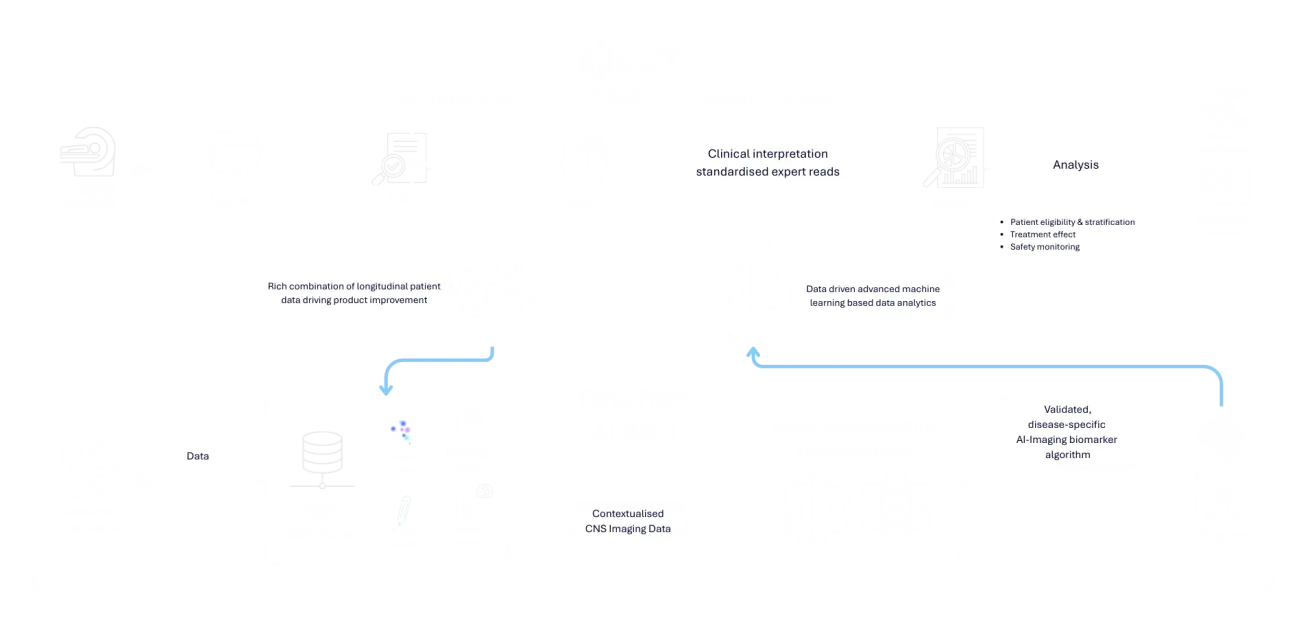
IXI™ facilitates AI-powered biomarker discovery, validation, and analysis, enabling deeper insights into disease progression, treatment response, and patient stratification.
Key Capabilities:
Automates complex image processing tasks, improving speed, accuracy, and reproducibility.
Proven infrastructure supporting multi-site, multi-country trials with centralised oversight.
IXI™ ensures the compliance required by regulatory agencies together with best practice industry standards such as ICH-GCP, 21 CFR Part 11, ISO-13485 and ISO/EC-27001.
Adaptable to sponsor needs, therapeutic areas, and trial phases.
Compatible with sponsor systems and workflows, requiring no additional software.
| End-to-End Workflow | From trial design and imaging site setup to data upload, quality control, and regulatory-compliant transfer, the IXI™ Platform ensures seamless clinical trial execution. |
| IXI™ Platform Interface | A secure, cloud-based portal for real-time image tracking, upload, pseudonymization, and query resolution across thousands of imaging sites worldwide. |
| Automated Quality Control & Scan Validation | Automated tools within the IXI Platform assess scan quality, protocol compliance, and standardisation, ensuring imaging data is trial-ready and regulatory-grade. |
| Centralised Radiology Reads | Flexible read workflows and bespoke read forms support blinded, multi-reader, and adjudicated reads—tailored to each protocol and therapeutic area. |
| Global Imaging Site Network | Over 2,000 qualified imaging sites across 75+ countries, trained and supported by IXICO’s expert teams. |
| Regulatory Compliance | Fully aligned with GCP, ISO13485, ISO/IEC27001, and CFR Part 11 standards. |
| Patient-Centric Trial Management | Tailored protocols and support for neurological patient populations, ensuring ease of participation and high-quality data capture. |

The IXI™ Platform continues to evolve, expanding its capabilities beyond clinical trials into diagnostics, real-world evidence, and AI-enhanced clinical decision support. With a foundation in neuroscience and a commitment to innovation, IXICO is shaping the future of neuroimaging through:
Including fluid biomarkers and hybrid imaging modalities.
Supporting earlier diagnosis, patient stratification, and predictive analytics through analysis automation.
Enabling targeted therapies and individualised treatment monitoring.
A flexible architecture that integrates bespoke workflows to deliver imaging endpoints with AI automated analysis tools and third-party pipelines.
Reach out to our team to learn more about IXI™, and discover how AI-powered neuroimaging can accelerate your research.
Contact Us