Early Phase Clinical Development
Delivering neuroimaging and digital biomarker solutions in early phase clinical development

Safety monitoring
We understand the importance of rigorous safety monitoring, with teams of expert neuroradiologists providing centralised real time reporting.
Radiological Reads
Scientific excellence
IXICO's clinical and imaging teams are experts in optimising the design and operational delivery of early phase clinical development.
Our collaborations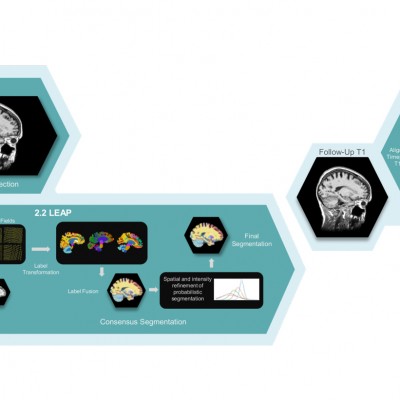
Staying flexible
We have a portfolio of image and biomarker analysis pipelines and also a range of tools to help develop and validate novel advanced algorithms.
IXICO are unique in their extensive validation of therapeutic area-specific advanced imaging endpoints, which we would not find elsewhere.